The Translational Research Pathway to Maximize University IP Value
Universities are hubs for groundbreaking medical technology innovation, but transforming these discoveries into patient-ready products is a formidable challenge. The journey from lab to market is often fraught with regulatory hurdles, undefined clinical pathways, and a critical "valley of death" where promising ideas can falter. This blog post explores how an integrated strategy, focusing on early regulatory engagement and a clear clinical development roadmap, can de-risk university MedTech, accelerate innovation, and maximize the value of intellectual property.
I. Bridging the “Valley of Death”
University commercialization efforts aim to turn academic discoveries into products that reach patients. The medical device pathway is complex, typically taking 3–7 years, and regulatory uncertainty can erode institutional clarity
Tech Transfer Office (TTO) Challenges
TTOs protect IP and accelerate licensing. Success depends-in part- on Translational Velocity—the speed from lab concept to market launch. Regulatory clarity builds onIP claims and allows for a faster, more efficient spinout
Innovation Fund Perspective
Early-stage MedTech risks— MVPs with no early Voice of Customer feedback, weak IP, and an undefined regulatory strategy—inflate costs. Integrated regulatory and clinical solutions, like MedTech Impact Partners’ CRO model, accelerate development, reduce capital burn, and optimize ROI.
II. Deconstructing Translational Gaps
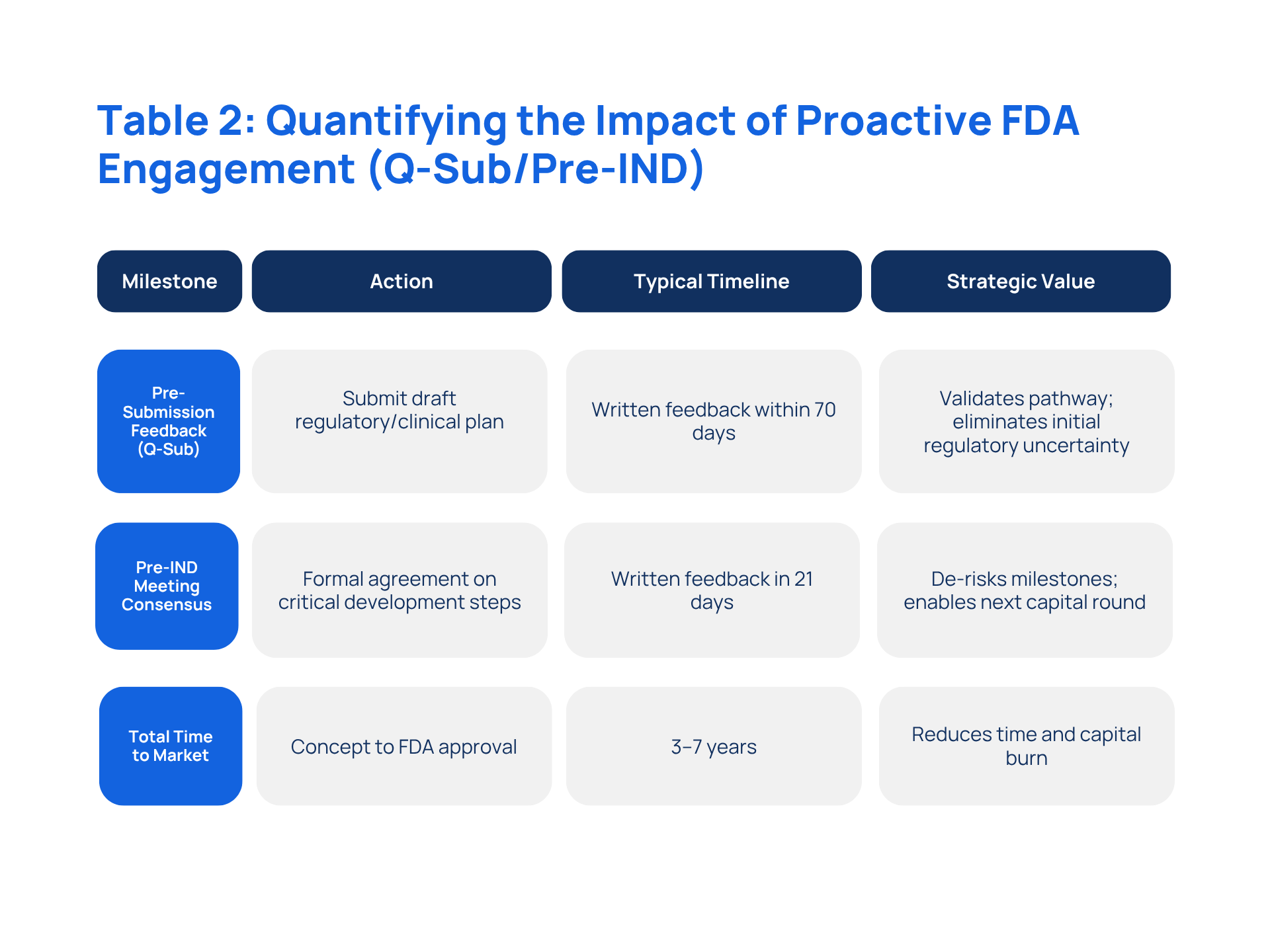
University spinouts can fail due to regulatory uncertainty, MVPs with little VOC, or non-defensible IP. Early FDA engagement through Pre-Submission (Q-Sub) or Pre-IND meetings provides written feedback, validates clinical plans, and accelerates Translational Velocity.
III. Case Study: AI-Based Device
A university team developing an AI-based imaging device faced limited regulatory expertise and grant budgets. MedTech Impact Partners provided a tailored regulatory strategy and pre-submission guidance:
Accelerated FDA approval by 30%
Saved ~$1M in potential trial costs
Secured full FDA agreement on critical development steps
This converted regulatory uncertainty into a tangible asset, unlocking funding and speeding market entry.
IV. Strategic Returns for Stakeholders
TTO: Faster case processing, defensible IP, stronger licensing leverage
Innovation Fund: Reduced capital risk, efficient follow-on funding
Center for Innovation: Repeatable framework for de-risking development
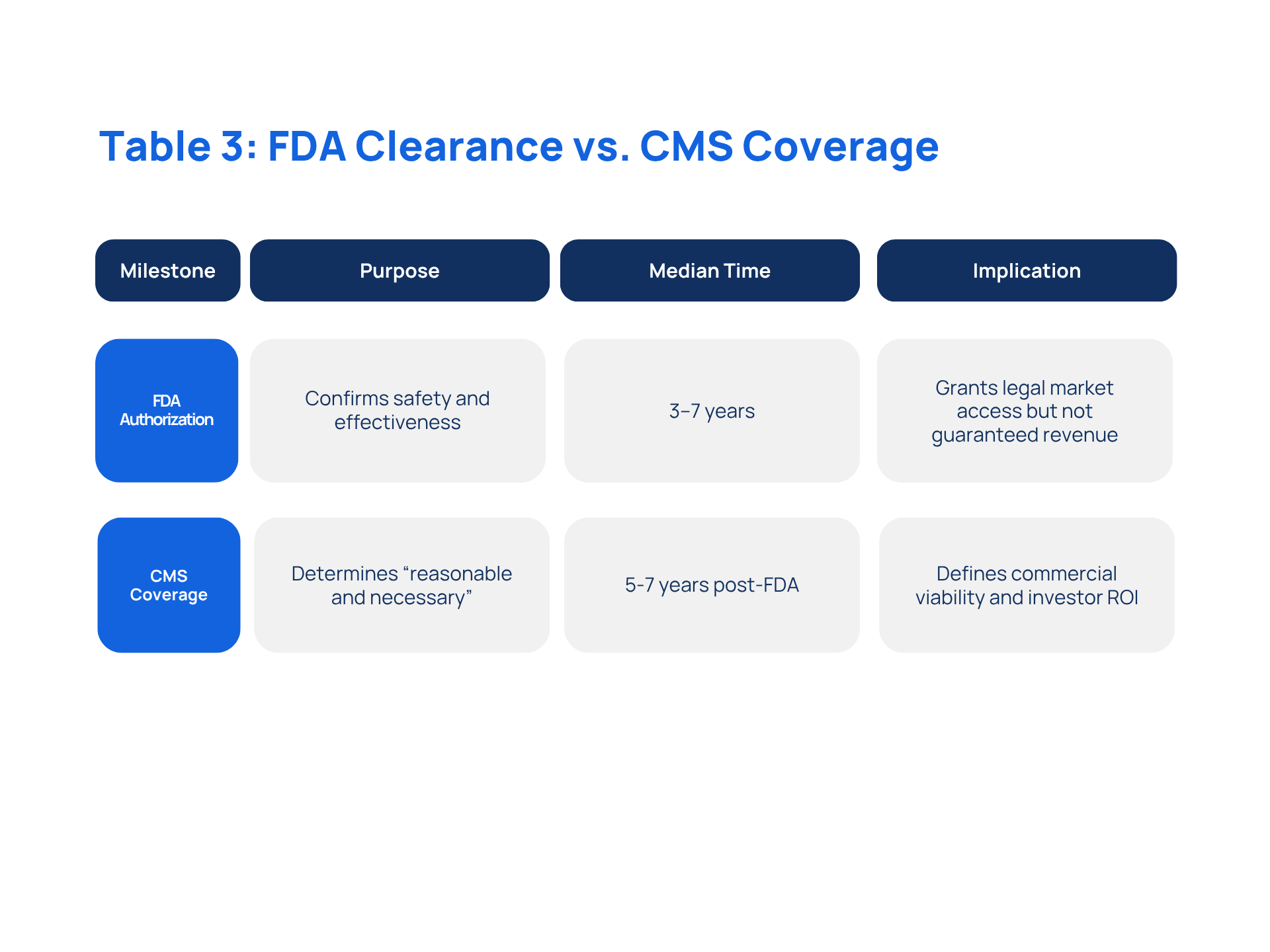
V. Preparing for Reimbursement
FDA clearance confirms safety but not revenue. CMS coverage can take 5+ years post-FDA authorization. Clinical strategies should target both FDA and CMS requirements, leveraging programs like the CMS Transformative Coverage Pathway for Emerging Technologies (TCET) to shorten the revenue gap.
VI. Conclusion
Translational Velocity—the speed at which technology navigates IP, regulatory, clinical and reimbursement pathways—is critical to university MedTech success. Proactive, integrated regulatory and clinical strategies maximize IP value, accelerate market entry, and create repeatable frameworks for transformative institutional impact.